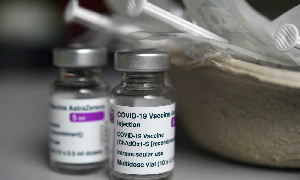- Home - News
- Polls
- Year In Review
- News Archive
- Crime & Punishment
- Politics
- Regional
- Editorial
- Health
- Ghanaians Abroad
- Tabloid
- Africa
- Religion
- Election 2020
- Coronavirus
- Photo Archives
- News Headlines
- Press Release
General News of Saturday, 11 September 2021
Source: ghana.dubawa.org
Gifting or dumping? Germany’s gift of 1.5 million AstraZeneca COVID-19 vaccines to Ghana
Germany in March 2021 suspended the use of the Oxford/AstraZeneca COVID-19 vaccine, owing to incidents of blood clotting in people below age 60. This decision came about after the country’s medicine regulator, The Paul Ehrlich Institute, found 31 cases of a rare type of blood clot in people vaccinated with the vaccine.
Fast forward to August 2021, five months after this suspension, the German government is set to give the Ghanaian government 1.5 million doses of the AstraZeneca COVID-19 vaccine.
This, according to the Director of Communication at the Presidency, Eugene Arhin, is as a result of discussions between The President, Akufo-Addo and the German Chancellor, Angela Merkel, following Germany’s offer to Africa of up to 70 million COVID-19 vaccine doses.
Does Germany’s suspension of the AstraZeneca COVID-19 vaccine have any play in their release of 70 million doses to Africa, of which 1.5 million is coming to Ghana?
Well, some social media users seem to think so.
Following the announcement of Ghana’s gift of 1.5 million doses of the vaccine, some social media users have questioned the intent behind the gesture with posts like the one below being seen online.
A March 12, 2021 report by Aljazeera has listed countries that have stopped using the AstraZeneca Covid-19 vaccine, listing Germany among the countries cited. The report, which states that over a dozen countries, mostly located in Europe, have held-off the use of the vaccine in states that Germany did the same as a precautionary measure while investigations were carried out of the cases involving the blood clots.
In May, 2021, however, Germany opened up AstraZeneca COVID-19 vaccines for all adults. This was after the suspension, which disallowed adults below 60-years from taking the shots, was lifted, indicating its safety for use. This decision was arrived at between the German federal and state officials as they concluded that the shot had far higher benefits than risks.
WHO’s take on Oxford/AstraZeneca Covid-19 vaccine
The World Health Organisation has issued interim recommendations for the use of the vaccine with directives for use by health workers at high risk of exposure to the coronavirus and older people, including those aged 65 and above.
In the guidance document, updated July 30, 2021, this vaccine is intended for use by people 18 years and above, regardless of a very rare syndrome of blood clotting combined with low platelet counts with the majority of such cases having been recorded in the United Kingdom and The European Union countries.
In general, the WHO considers the AstraZeneca COVID-19 vaccine safe for use after it underwent SAGE consideration and the European Medicines Agency review.