Opinions of Monday, 9 December 2019
Columnist: Moses Alhassan
Nitrogen volatilization
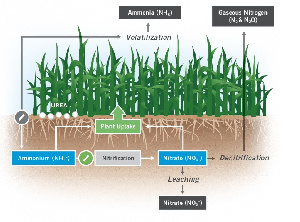
Nitrogen is quite stable in the atmosphere. However, its form in the soil can change very easily. Fertilizer nitrogen may be lost from the soil in several ways such as ammonia volatilization, nitrate leaching, and nitrate denitrification.
In a study by Jones et al. (2013), any surface applied ammonia and ammonium-based N fertilizer, including manure can lose N to the atmosphere. The potential is greatest with urea and fluids containing urea such as urea ammonium nitrate (UAN). In any case, what really causes nitrogen losses?
Urea represents more than 50% of the world’s nitrogenous fertilizers (Mandal et al., 2019) and being mostly prilled or granular, Urea is easy to store, transport and apply on agricultural fields. On the other hand, a significant amount of urea may be lost to the atmosphere as ammonia gas under specific conditions. In the hydrolysis of urea to unstable carbamic acid, urease (a naturally occurring enzyme) catalyzes this process. Carbamic acid then decomposes rapidly to form ammonia (which is most likely to escape to the atmosphere) and carbon dioxide. However, when ammonia reacts with water, ammonium (NH4+) is formed and this provides usable nitrogen to plants. Hydroxide ion is formed from this process and this increases the pH of the soil, making it more alkaline and consequently increasing the rate of ammonia volatilization within the region. Ammonia volatilization is greatly influenced by several factors.
Temperature, soil pH and moisture have been largely researched to be major factors that influence the volatilization of ammonia (Jones et al., 2013). On the other hand (though not so much researched), the source of fertilizer, residue content on field and time of application affects the rate at which nitrogen is lost to the atmosphere. For instance, Ammonia volatilization is most likely to take place when soils are moist, warm and the source of urea, near the surface of the soil. Alkaline soils (pH greater than 8) also foster ammonia volatilization as well. Is it possible to mitigate or minimize nitrogen volatilization?
To begin with, applying fertilizer from the right source at the right rate, at the right time and in the right place initiates a good pathway for high and quality yields. Nevertheless, urease inhibitors also play a crucial role in reducing nitrogen losses as they are used to temporarily reduce the activity of the enzyme and slow the rate at which urea is hydrolyzed. Yara International, being a leading producer of nitrogen fertilizers has also made substantive contributions in mitigating nitrogen volatilization.
Yara’s efforts to feed the world, and protect the planet has led to the development of various crop nutrition products that have been adequately tested, tried and experimented by independent universities and research institutions across the world. Among these product ranges is YaraVera (Yara’s range of improved urea). YaraVera AMIDAS contains 40% of nitrogen and 5.6% of Sulphur. This product has been well researched and used by local farmers (both commercial and small-scale) to reflect its ability to improve nitrogen efficiency from urea by reducing nitrogen volatilization losses up to 35% on low pH soils (Yara, 2017). Additionally, there is YaraVera AMIPLUS which contains 46% N and NBPT (urease inhibitor) aids in decreasing nitrogen losses and increasing urea effectiveness. These are sustainable innovations that are helping farmers around the world to achieve appreciable and quality yields.
References
Jones, C., Brown, B. D., Engel, R., Horneck, D., & Olson-Rutz, K. (2013). Nitrogen Fertilizer Volatilization Factors Affecting.
Mandal, S., Donner, E., Smith, E., Sarkar, B., & Lombi, E. (2019). Biochar with near-neutral pH reduces ammonia volatilization and improves plant growth in a soil-plant system: A closed chamber experiment. Science of the Total Environment, 697, 134114. https://doi.org/10.1016/j.scitotenv.2019.134114 Yara. (2017).
Yara Fertilizer Industry Handbook. Oslo.
Entertainment